[Principle] In addition to trypsin, which is found in the pancreas of animals, there are two other proteolytic enzymes with similar properties: chymotrypsin and elastase. During the extraction process, these three enzymes are difficult to separate due to their structural and functional similarities. Therefore, advanced methods are required to further isolate and purify them for specific applications. The extraction of trypsin typically begins by isolating trypsinogen from pancreatic cells using a dilute acid solution. Once extracted, the pH of the solution is adjusted to an acidic level (around 3.0) based on the principle of isoelectric precipitation, causing the precipitation of various acidic proteins, including trypsinogen, chymotrypsinogen, and elastase. These precipitates are then redissolved in water and the pH is raised to around 8.0. A small amount of active trypsin is added to activate the zymogens, allowing them to convert into their active forms. This activation process involves complex interactions between the three zymogens, resulting in the formation of active enzymes. [Reagents and Equipment] 1. Reagents (1) Acetic acid solution with a pH of 2.5–3.0 (2) 10% acetic acid (by volume) (3) 2 mol/L sulfuric acid (4) Solid ammonium sulfate (5) Anhydrous calcium chloride (6) Crystalline trypsin (7) 25% ethanol solution containing 0.015 mol/L HCl and 0.05 mol/L CaCl₂ (8) 95% ethanol (by volume) (9) 5 mol/L sodium hydroxide (10) Acetone 2. Equipment (1) Fresh animal pancreas (2) Tissue homogenizer (3) Centrifuge (4) Magnetic stirrer (5) Dialysis bag, 20-mesh sieve, gauze, water bath (6) Beakers, measuring cylinders, pipettes, test tubes, glass funnels (7) Buchner funnel, suction flask, thermometer, dropper, magnetic stir bar, gauze, pH paper, etc. [Methods and Procedures] Method One 1. Extraction of Trypsinogen Begin with approximately 150 g of fresh pancreas. Remove connective tissue and fat, then take 100 g of the clean tissue. Cut it into small pieces and mash it using a tissue homogenizer. Add twice the volume of pre-cooled acetic acid (pH 2.5–3.0) and mix thoroughly. Transfer the slurry to a 500 mL beaker and adjust the pH to 2.5–3.0 using 10% acetic acid. Extract at 5–10°C for more than 6 hours, stirring occasionally. Filter through four layers of gauze and collect the filtrate. Repeat the extraction once more with 0.5 times the volume of pre-cooled acetic acid. Combine both filtrates, adjust the pH to 2.5–3.0 using 2.5 mol/L sulfuric acid, and let it stand at 4°C for 4 hours to allow precipitation of acidic proteins. Filter through folded filter paper, measure the volume (about 200 mL), and add solid ammonium sulfate to reach 75% saturation. Let it sit overnight and perform suction filtration to collect the crude trypsinogen. 2. Activation of Trypsinogen Weigh the crude trypsinogen and dissolve it in 10 times its weight in pre-cooled distilled water. Adjust the pH to 8.0 using 5 mol/L NaOH and add anhydrous CaCl₂ to achieve a final Ca²+ concentration of 0.1 mol/L. Take a 2 mL sample to measure protein content and enzyme activity before activation. Add 5 mg of crystalline trypsin to the solution and gently stir. Incubate at 25°C for 2–4 hours or at 4°C for 12–16 hours. Monitor the enzyme activity every hour until the rate slows down. After activation, measure the protein content and enzyme activity again. Adjust the pH to 2.5–3.0 using 2 mol/L sulfuric acid, filter to remove calcium sulfate, and store the solution at 4°C for later use. Method Two Using 100 g of frozen pig pancreas, thaw it at 2–5°C and cut it into small pieces. Use a tissue homogenizer to prepare pancreatic juice and store it at 5–10°C for over 24 hours. Add 250 mL of 25% ethanol solution (containing 0.015 mol/L HCl and 0.05 mol/L CaCl₂) and mix for 1 minute. Pour the mixture into a 500 mL beaker and stir intermittently at about 20°C for 5–6 hours. At 1.5 hours, take 2 mL of the solution to measure protein and enzyme activity after activation. After the reaction is complete, centrifuge at 3,500 rpm for 20 minutes, filter the supernatant through two layers of gauze, and measure the volume. Add powdered ammonium sulfate to reach 20% saturation, let it stand for 6 hours, and centrifuge again. Store the supernatant for further processing. Wash the precipitate with 95% ethanol and acetone, filter, and dry it to obtain crude trypsin I. For the second stage, add more ammonium sulfate to the supernatant to reach 55% saturation, let it stand for 6 hours, and centrifuge at 3,500 rpm for 30 minutes. Wash the precipitate with ethanol and acetone, filter, and dry to obtain crude trypsin II. Foshan Gruwill Hardware Products Co., Ltd. , https://www.zsgruwill.com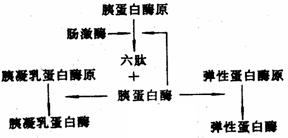 Another method involves using a solution with an appropriate pH to promote the autolysis of trypsinogen. By introducing Ca²+ ions into the solution, the trypsinogen can begin to self-digest in a controlled manner, especially when activated by a small quantity of trypsin. This process also activates chymotrypsinogen and elastase, enabling the production of active enzymes. After this step, the activated enzyme solution undergoes additional purification techniques to ensure high purity and activity.
Another method involves using a solution with an appropriate pH to promote the autolysis of trypsinogen. By introducing Ca²+ ions into the solution, the trypsinogen can begin to self-digest in a controlled manner, especially when activated by a small quantity of trypsin. This process also activates chymotrypsinogen and elastase, enabling the production of active enzymes. After this step, the activated enzyme solution undergoes additional purification techniques to ensure high purity and activity.